Fermentation is an essential biological process where organisms convert carbohydrates into energy in the absence of oxygen. Understanding the environmental factors that influence fermentation is crucial not only for biological theory but also for industrial applications such as baking, brewing, and biofuel production. This investigation focuses on the effect of salinity on the metabolic activity of yeast.
To what extent does increasing the concentration of sodium chloride (0.0%, 1.0%, 2.0%, 3.0%, 4.0% w/v) affect the rate of fermentation in Saccharomyces cerevisiae, as measured by the volume of carbon dioxide displaced (±0.5 cm3) over a 5-minute and 10-minute period at 45°C?
Saccharomyces cerevisiae, commonly known as baker's yeast, is a facultative anaerobe. When oxygen is unavailable, it performs alcoholic fermentation, converting glucose (C6H12O6) into ethanol (2C2H5OH), carbon dioxide (2CO2), and energy (ATP). The overall equation is:
C6H12O6 → 2C2H5OH + 2CO2 + 2ATP
The rate of this reaction is governed by enzymes such as zymase. Enzyme activity is sensitive to environmental conditions including temperature, pH, and ion concentration. Sodium chloride (NaCl) introduces two main stress factors: osmotic stress and specific ion toxicity.
Osmotic Stress: The addition of NaCl lowers the solute potential (-Ψs) and thus the total water potential (Ψ) of the extracellular environment. If the external water potential drops below that of the yeast cytoplasm, water moves out of the cell via osmosis. This loss of turgor pressure can lead to cell shrinkage (plasmolysis), reducing the availability of free water required for metabolic hydrolysis reactions.
Specific Ion Toxicity: High concentrations of sodium (Na+) and chloride (Cl-) ions can penetrate the cell wall and plasma membrane. These ions can disrupt the ionic bonds and hydrogen bonds that maintain the tertiary structure of enzymes. This denaturation alters the shape of the active site, preventing the substrate (glucose) from binding, thereby inhibiting glycolysis and fermentation (Belz et al., 2017).
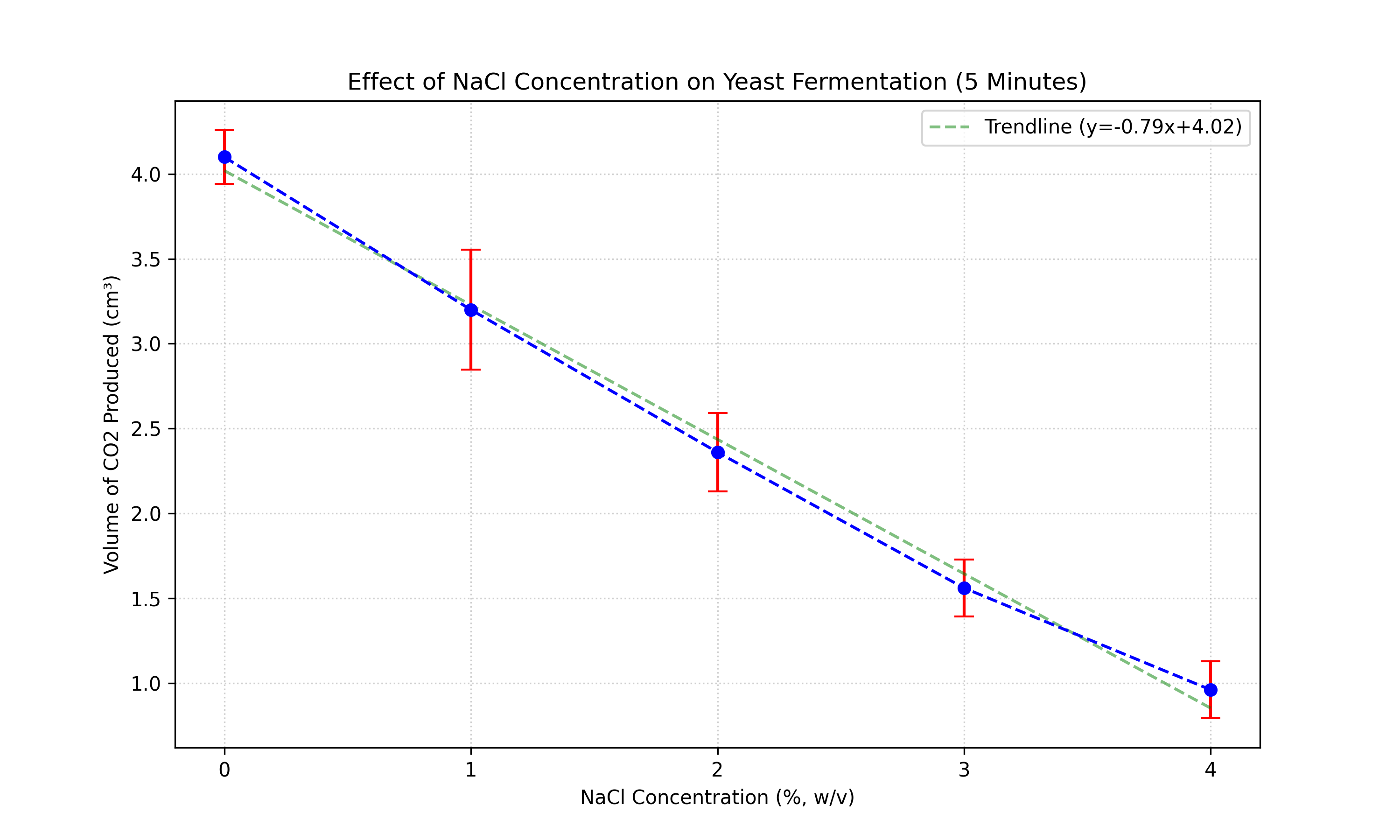
| NaCl Conc. (%) | Trial 1 | Trial 2 | Trial 3 | Trial 4 | Trial 5 | Mean (cm3) | Std Dev (cm3) |
|---|---|---|---|---|---|---|---|
| 0 | 4.0 | 3.9 | 4.3 | 4.1 | 4.2 | 4.10 | 0.16 |
| 1 | 3.2 | 3.0 | 3.8 | 2.9 | 3.1 | 3.20 | 0.35 |
| 2 | 2.4 | 2.7 | 2.4 | 2.1 | 2.2 | 2.36 | 0.23 |
| 3 | 1.6 | 1.6 | 1.4 | 1.8 | 1.4 | 1.56 | 0.17 |
| 4 | 1.0 | 0.8 | 1.2 | 0.8 | 1.0 | 0.96 | 0.17 |
One-Way ANOVA (5 Minutes):
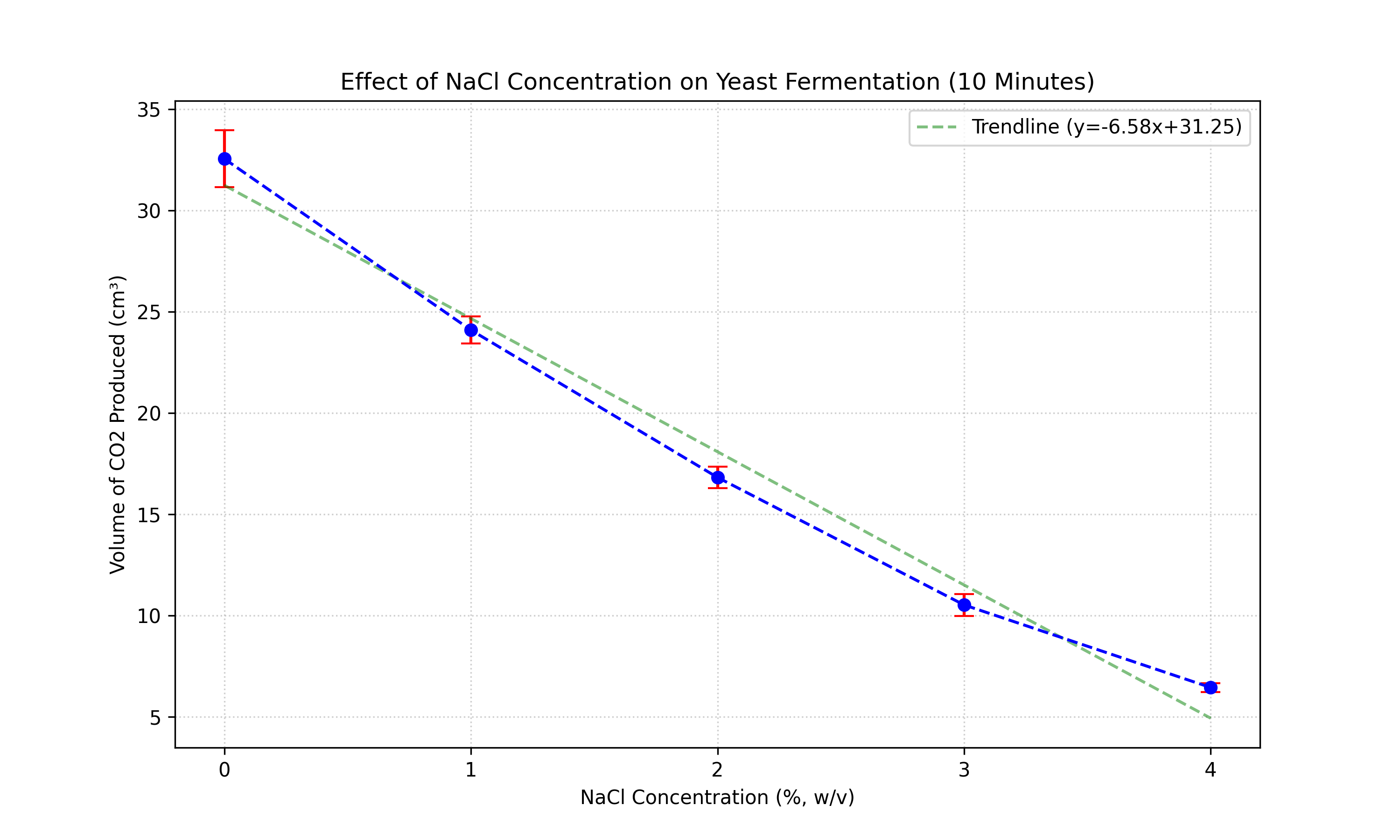
| NaCl Conc. (%) | Trial 1 | Trial 2 | Trial 3 | Trial 4 | Trial 5 | Mean (cm3) | Std Dev (cm3) |
|---|---|---|---|---|---|---|---|
| 0 | 32.0 | 30.5 | 34.2 | 32.7 | 33.4 | 32.56 | 1.41 |
| 1 | 24.0 | 23.8 | 25.2 | 23.4 | 24.1 | 24.10 | 0.67 |
| 2 | 17.0 | 17.6 | 16.8 | 16.3 | 16.4 | 16.82 | 0.51 |
| 3 | 10.5 | 11.0 | 9.8 | 11.1 | 10.2 | 10.52 | 0.53 |
| 4 | 6.5 | 6.4 | 6.5 | 6.1 | 6.7 | 6.44 | 0.22 |
One-Way ANOVA (10 Minutes):
Both datasets show a clear negative trend: as salt concentration increases, CO2 production decreases. The inhibition is evident early on (5 mins) and persists over time (10 mins).
However, the F-statistic increases dramatically from 152.13 (5 min) to 898.98 (10 min). This suggests that the divergence between the groups becomes more distinct over time. In the first 5 minutes, there might be a "lag phase" or initial thermal expansion masking the full extent of fermentation inhibition. By 10 minutes, the cumulative effect of the lower metabolic rate in high-salt conditions results in a much clearer separation of data points, reducing relative error (noise) compared to the signal (treatment effect).
The results strictly support the research hypothesis. There is a strong, negative correlation between NaCl concentration and yeast fermentation rate at both time intervals. The statistical analysis (ANOVA) confirms that the probability of these results occurring by chance is virtually zero (p < 0.001).
The biological mechanism is confirmed: the hypertonic solution caused by increased NaCl led to osmotic water loss from the yeast cells, while potential Na+ and Cl- ion toxicity disrupted enzyme function, cumulatively leading to the observed reduction in CO2 output.
Strengths: The high F-statistics indicate a very strong treatment effect compared to random error, validating the experimental design's ability to detect changes. The consistent small standard deviations (error bars) further demonstrate precise control of variables.
Limitations: